U.S. Degenerative Disc Disease Treatment Market Size Projected to Reach USD 6.10 Billion by 2032
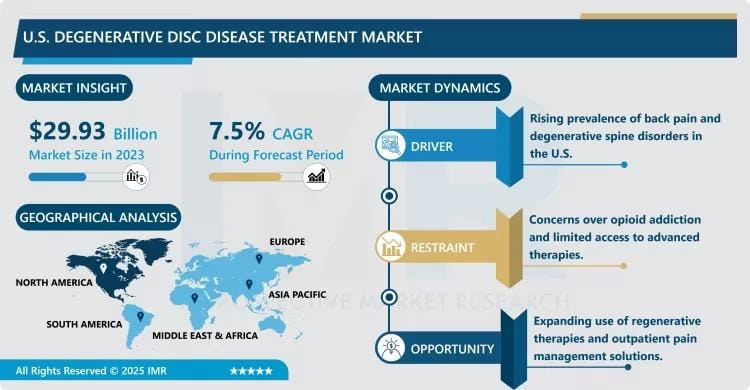
The U.S. Degenerative Disc Disease Treatment Market is a critical vertical within the orthopedic and neurosurgical landscape, focusing on therapeutic interventions for the gradual deterioration of intervertebral discs. This condition is a leading cause of chronic lower back and neck pain in the American population, often resulting from age-related wear and tear, obesity, or occupational strain. The market encompasses a wide spectrum of care, from conservative non-surgical management including specialized pharmacotherapy (NSAIDs, muscle relaxants) and physical therapy, to advanced surgical solutions such as spinal fusion, artificial disc replacement, and neuromodulation.
The primary advantage of modern DDD treatments over traditional methods lies in the shift toward motion-preserving and minimally invasive surgical (MIS) techniques. Unlike older, more invasive procedures that often resulted in long recovery times and adjacent segment disease, contemporary solutions like total disc replacement and dynamic stabilization allow for improved patient mobility and reduced post-operative complications. These advancements are predominantly utilized in hospitals and rapidly expanding ambulatory surgical centers (ASCs), providing a higher quality of life for millions of Americans suffering from debilitating spinal conditions.
Market Segmentation:
The U.S. Degenerative Disc Disease Treatment Market is segmented into Product Type, Application, and End User. By Product Type, the market is categorized into Drugs (Acetaminophen, NSAIDs, Muscle Relaxants, Steroids) and Devices (Spinal Fusion Devices, Motion Preserving Devices, Spinal Traction & Decompression Systems, Neuromodulation Devices). By Application, the market is categorized into Lumbar Spine, Cervical Spine, and Thoracic Spine. By End User, the market is categorized into Hospitals, Specialty & Orthopedic Clinics, and Ambulatory Surgical Centers (ASCs).
Growth Driver:
Rapidly Aging Population and High Prevalence of Sedentary Lifestyles: The primary driver for the U.S. DDD treatment market is the significant demographic shift toward an older population. According to U.S. Census data, the geriatric segment is expanding at an unprecedented rate, with older adults being significantly more susceptible to spinal disc degeneration. Furthermore, the modern American lifestyle—characterized by prolonged sitting, sedentary behavior, and rising obesity rates—has led to a higher incidence of early-onset degenerative conditions. This growing patient pool creates a consistent and rising demand for both long-term pain management and corrective surgical interventions.
Market Opportunity:
Advancements in Regenerative Medicine and Cell-Based Therapies: A major opportunity exists in the commercialization of regenerative therapies, such as stem cell injections and Platelet-Rich Plasma (PRP) treatments, aimed at reversing disc damage rather than just managing symptoms. As the FDA streamlines pathways for breakthrough regenerative products, there is a substantial opening for "biological" solutions that could delay or eliminate the need for invasive fusion surgeries. Companies investing in injectable disc cell therapies (IDCT) stand to capture a massive market share of patients who prefer non-permanent, biological restoration over mechanical implants.
Detailed Segmentation:
U.S. Degenerative Disc Disease Treatment Market, Segmentation The U.S. Degenerative Disc Disease Treatment Market is segmented on the basis of Product Type, Application, and End User.
Product Type The Product Type segment is further classified into Drugs and Devices. Among these, the Devices sub-segment accounted for the highest market share in 2024, representing over 60% of the market. This dominance is attributed to the high unit cost and frequent use of spinal implants, fusion cages, and artificial discs in surgical procedures. The steady trend toward motion-preserving technologies and 3D-printed porous implants has further solidified this segment's lead as surgeons opt for advanced hardware that promotes faster bone in-growth and long-term stability.
Application The Application segment is further classified into Lumbar Spine, Cervical Spine, and Thoracic Spine. Among these, the Lumbar Spine sub-segment accounted for the highest market share in 2024, contributing to over 50% of the total revenue. The lumbar region bears the majority of the body's weight and is the most common site for mechanical stress, making it the primary focus for degenerative conditions. The high volume of lumbar fusion and discectomy procedures performed annually in the U.S. ensures that this segment remains the most lucrative part of the market.
Some of The Leading/Active Market Players Are-
- Medtronic plc (Ireland/USA)
- Johnson & Johnson Services, Inc. (DePuy Synthes) (USA)
- Stryker Corporation (USA)
- Zimmer Biomet Holdings, Inc. (USA)
- Globus Medical, Inc. (USA)
- Alphatec Holdings, Inc. (ATEC) (USA)
- Orthofix US LLC (USA)
- B. Braun Medical Inc. (Germany/USA)
- Abbott Laboratories (USA)
- NuVasive, Inc. (USA)
- DiscGenics, Inc. (USA)
- Centinel Spine, LLC (USA)
- Companion Spine (USA/France)
- Spine Wave, Inc. (USA)
- Zavation Medical Products, LLC (USA)
- and other active players.
Key Industry Developments
News 1: In December 2025, Companion Spine announced that the U.S. FDA granted Premarket Approval (PMA) for the DIAM™ Spinal Stabilization System. This is the first posterior motion-preserving device approved in the U.S. for treating moderate to severe low back pain secondary to DDD. The approval is a landmark for the industry, offering a reversible, minimally invasive alternative to spinal fusion. By preserving segmental mobility and leaving future surgical options open, this technology addresses an underserved population of patients who fail conservative care but are not yet candidates for more aggressive reconstruction.
News 2: In February 2025, Alphatec (ATEC) unveiled significant advancements in their Prone Transpsoas (PTP) technique. The update extends the use of this minimally invasive corpectomy approach to more complex cases of Degenerative Disc Disease. This development reflects the ongoing market shift toward "Prone" surgery, which allows for better spinal alignment and faster patient recovery. By refining specialized instrumentation for complex DDD cases, ATEC continues to challenge the traditional "open" surgery paradigm, driving volume toward outpatient and specialized orthopedic centers.
Key Findings of the Study
- Hospitals remain the largest end-user, accounting for 55% of the market share, though Ambulatory Surgical Centers (ASCs) are the fastest-growing segment.
- Motion-preserving devices are seeing higher adoption rates compared to traditional fusion, as patients demand faster return-to-activity.
- The Lumbar Spine application continues to dominate, driven by high rates of lower-back pain diagnoses in the aging U.S. workforce.
- FDA Breakthrough Designations for regenerative cell therapies are expected to disrupt the traditional device-heavy market structure by 2030.
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the U.S. Degenerative Disc Disease Treatment Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global Orthopedic and Spine Care industry.
📞 Contact Us
Introspective Market Research Pvt. Ltd.
Phone: +91-91753-37569
Email: sales@introspectivemarketresearch.com
Web: www.introspectivemarketresearch.com
- Secret Key on-line
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- Art
- Life
- Coding




