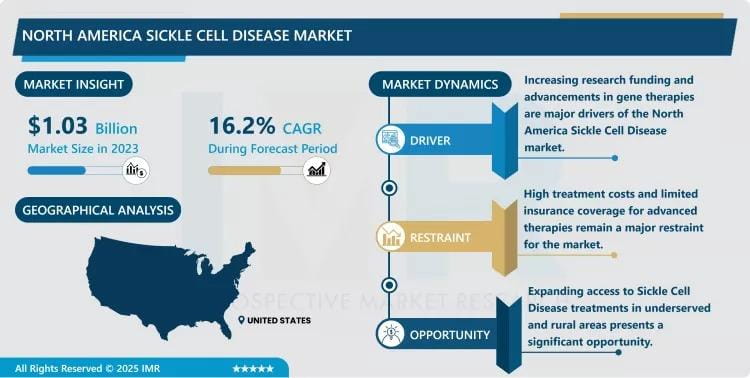
North America Sickle Cell Disease Market Size Projected to Reach USD 4.01 Billion by 2032
The North America Sickle Cell Disease Market encompasses the medical treatments, diagnostics, and patient management services for this inherited blood disorder, which is characterized by abnormally shaped red blood cells. SCD leads to chronic anemia, debilitating vaso-occlusive crises (VOCs), and severe organ damage. This market is undergoing a profound transformation driven by the shift from solely symptomatic management to disease-modifying and curative therapies.
The treatment landscape has dramatically evolved beyond conventional blood transfusions and hydroxyurea. Recent advancements, particularly the introduction of novel drugs like voxelotor and crizanlizumab, and, most notably, the regulatory approval of gene therapies (such as exa-cel and lovo-cel), offer the prospect of a functional cure. North America, dominated by the US, benefits from a well-established healthcare infrastructure, high awareness, and significant public and private investment in rare disease research, making it the leading market for the adoption of these complex, high-cost, and life-changing treatments.
Market Segmentation:
The North America Sickle Cell Disease Market is segmented into Treatment, Disease Type, and Distribution Channel. By Treatment, the market is categorized into Pharmacotherapy (Hydroxyurea, Voxelotor, Crizanlizumab, and L-glutamine), Blood Transfusion, and Bone Marrow/Stem Cell Transplant (including Gene Therapies). By Disease Type, the market is categorized into Sickle Cell Anemia (HbSS), Sickle Beta Thalassemia, and Sickle Hemoglobin C Disease (HbSC). By Distribution Channel, the market is categorized into Hospitals, Specialty Clinics, and Other End-Users (Homecare).
Growth Driver:
Regulatory Approval and Launch of Curative Gene Therapies and Disease-Modifying Drugs: The major growth driver is the US FDA approval and subsequent commercial launch of groundbreaking, one-time curative gene therapies, such as CASGEVY™ (exa-cel) and LYFGENIA™ (lovo-cel). These therapies, which correct the underlying genetic defect, command premium pricing and represent a massive revenue influx into the market. Concurrently, the uptake of disease-modifying agents like Oxbryta (voxelotor) and Adakveo (crizanlizumab) for crisis prevention further boosts pharmacotherapy revenues and underscores the market's decisive shift toward high-value, targeted interventions.
Market Opportunity:
Expansion of Newborn Screening Programs and Federal Funding for Access: A key market opportunity is the continued expansion and optimization of newborn screening programs across all US states, coupled with increasing federal and state efforts to improve patient access and reimbursement. Policies like the U.S. Centers for Medicare & Medicaid Services' Cell and Gene Therapy Access Model are designed to make high-cost curative treatments more accessible to the underserved patient population. This focus on health equity and early diagnosis ensures a steady pipeline of eligible patients for both conventional and advanced therapies, dramatically expanding the long-term potential of the North American market.
Detailed Segmentation:
North America Sickle Cell Disease Market, Segmentation The North America Sickle Cell Disease Market is segmented on the basis of Treatment, Disease Type, and Distribution Channel.
Treatment The Treatment segment is further classified into Pharmacotherapy, Blood Transfusion, and Bone Marrow/Stem Cell Transplant. Among these, the Pharmacotherapy sub-segment is expected to grow at the fastest CAGR during the forecast period. The high growth in pharmacotherapy is driven by the steady commercial success and high adoption rates of recently approved branded disease-modifying drugs like voxelotor, crizanlizumab, and L-glutamine, which are used chronically to prevent complications and improve patient quality of life.
Disease Type The Disease Type segment is further classified into Sickle Cell Anemia (HbSS), Sickle Beta Thalassemia, and Sickle Hemoglobin C Disease (HbSC). Among these, the Sickle Cell Anemia (HbSS) sub-segment accounted for the highest market share in 2024. HbSS, being the most severe and prevalent form of SCD in North America, drives the largest volume of demand across all treatment modalities, including routine blood transfusions, chronic pharmacotherapy, and the highly complex and expensive curative procedures.
Some of The Leading/Active Market Players Are-
- Pfizer Inc. (Global Blood Therapeutics) (USA)
- Novartis AG (Switzerland)
- Vertex Pharmaceuticals Incorporated (USA)
- CRISPR Therapeutics AG (Switzerland)
- bluebird bio, Inc. (USA)
- Emmaus Life Sciences, Inc. (USA)
- Bristol Myers Squibb Company (USA)
- Sanofi SA (France)
- Agios Pharmaceuticals, Inc. (USA)
- Global Blood Therapeutics (Acquired by Pfizer) (USA)
- Addmedica (France)
- and other active players.
Key Industry Developments
News 1: In December 2023, the U.S. FDA granted approval to two landmark gene therapies, CASGEVY™ (exa-cel by Vertex/CRISPR) and LYFGENIA™ (lovo-cel by bluebird bio), for treating sickle cell disease in eligible patients aged 12 and older. These approvals marked a pivotal moment, transforming SCD from a managed chronic illness into a potentially curable genetic disease and immediately establishing a new, ultra-high-cost tier in the treatment market, which will shape future reimbursement models.
News 2: In August 2025, the Centers for Medicare & Medicaid Services (CMS) finalized the framework for the Cell and Gene Therapy Access Model to improve payment predictability and access for curative SCD treatments in the Medicaid population. This decisive policy action addresses the major challenge of affordability for multi-million-dollar therapies, creating a mechanism for state Medicaid programs to offer specialized care and substantially reducing financial barriers for a critical segment of the patient demographic.
Key Findings of the Study
- Sickle Cell Anemia (HbSS) remains the dominant disease type, driving the largest treatment volume.
- The United States accounts for the vast majority of the North American market share due to advanced infrastructure and high drug prices.
- The market is primarily driven by the FDA approval and commercialization of Gene Therapies and next-generation pharmacotherapies.
- A key market trend is the shift in reimbursement models toward value-based payment and outcome-linked contracts for curative treatments.
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the North America Sickle Cell Disease Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global Rare Disease and Gene Therapy industry.
📞 Contact Us Introspective Market Research Pvt. Ltd.
Phone: +91-91753-37569
Email: sales@introspectivemarketresearch.com
Web: www.introspectivemarketresearch.com
- Live Stream
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- Art
- Life
- Coding




